magnesium chloride dot diagram|High School Chemistry/Lewis Electron Dot Diagrams : Baguio #tutorials #chemistry #lessonsIn this Chemistry learning video, we will focus on drawing dot and cross diagram of ionic compound, magnesium chloride.Ace Chem. Security System, GF, Yuvallos ldg., D. Veloso Ave., rgy. Punta, Ormoc ity or thru the Admin Officer of the SSS ranch where the property/ies is/are located. 2. No prequalification documents shall be received after 12:01 PM of December .
magnesium chloride dot diagram,#tutorials #chemistry #lessonsIn this Chemistry learning video, we will focus on drawing dot and cross diagram of ionic compound, magnesium chloride.Ace Chem. A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses .

964. 128K views 5 years ago. A step-by-step explanation of how to draw the MgCl2 Lewis Dot Structure. For MgCl2 we have an ionic compound and we need to take that into account when we dra .more.magnesium chloride dot diagram964. 128K views 5 years ago. A step-by-step explanation of how to draw the MgCl2 Lewis Dot Structure. For MgCl2 we have an ionic compound and we need to take that into account when we dra .more.
Tutor 360. 36 subscribers. 9. 23 views 4 days ago SHORTS. Part 1: Breaking down how to draw the dot and cross diagram of Magnesium Chloride (MgCl2). Full .We’ll use Lewis Electron Dot Symbols to describe the ionic bonding that is observed in magnesium chloride. The formation of magnesium chloride can be thought of as a result from a reaction involving magnesium metal, . Magnesium is element #12. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1 s2 2 .
Here are some other examples of dot and cross diagrams for the formation of ions in some ionic compounds – magnesium oxide and calcium chloride. 1. Ionic bonding in .
Draw the electron configuration diagram for each atom. Draw the outer shell of each atom. Magnesium has two electrons in its outer shell, oxygen has six. Swap the crosses for dots in one of your .
The dot-and-cross diagrams of ionic compounds that we will be looking at will be these: sodium chloride. sodium oxide. magnesium chloride. magnesium oxide. aluminium oxide. more on dot-and-cross .

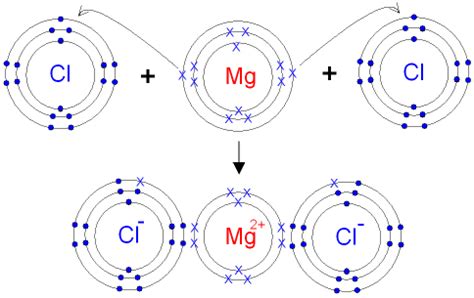
Dot and cross diagrams are diagrams that show the arrangement of the outer-shell electrons in an ionic or covalent compound or element. The electrons are shown as dots .Electronic configuration of Magnesium (atomic number = 12) is 2, 8, 2 and that of chlorine atom (atomic number = 17) is 2, 8, 7. So, magnesium will lose two electrons to each of the chlorine atom in order for every atom to complete its octet. Electron dot structure of magnesium chloride:- .Representing Dot & Cross Diagrams. Dot and cross diagrams are diagrams that show the arrangement of the outer-shell electrons in an ionic or covalent compound or element. The electrons are shown as dots and crosses. In a dot and cross diagram: Only the outer electrons are shown. The charge of the ion is spread evenly which is shown by using . How to draw the dot and cross diagram for magnesium chloride. GCSE Edexcel chemistry
Magnesium chloride is an inorganic compound with the formula Mg Cl 2. . (Pourbaix diagram). MgCl 2 → Mg + Cl 2. The production of metallic magnesium at the cathode (reduction reaction) is accompanied by the oxidation of the chloride anions at the anode with release of gaseous chlorine. This process is developed at a large industrial scale.
The Lewis dot diagram is a visual representation of the valence electrons of an atom. For magnesium, which has an atomic number of 12, the diagram shows two dots next to the element’s symbol, Mg. This indicates that magnesium has two valence electrons in its outermost energy level. The Lewis dot diagram of magnesium can be interpreted to .Here are some other examples of dot and cross diagrams for the formation of ions in some ionic compounds – magnesium oxide and calcium chloride. Image gallery Skip image gallery.Highly Recommended - Top Tutors for All Subjects at All Levels here: https://spires.co/franklychemistryThis brief flash video shows how atoms of magnesium an.High School Chemistry/Lewis Electron Dot Diagrams Dot-and-Cross Diagram 4 of Ionic Compound: Magnesium Oxide. To draw the dot-and-cross diagram of magnesium oxide, let’s first determine its formula. Since magnesium is in group II of the periodic table, it forms ions with charge of +2. Oxygen is in group VI of the periodic table, it forms ions with charge of -2.Ionic or electrovalent bonds are formed by mutual sharing of electrons between atoms. Loss of electrons is called Oxidation and gain of electron is called Reduction. The electrons which are not involved in bonding are called valence electrons. Discuss in brief about the properties of coordinate covalent compounds.A chlorine atom will gain an electron to form a negatively charged chloride ion with a charge of 1-Formula of ionic compound: . Magnesium Oxide Dot & Cross Diagram. Magnesium is a group 2 metal so will lose two outer electrons to another atom to have a full outer shell of electrons;
Here is the electron configuration for sodium. The electron configuration is: 1 s2 2 s2 2 p6 3 s1. The core electrons are 1 s2 2 s2 2 p6. The valence electron is 3 s1. One way to represent this valence electron, visually, was developed by Gilbert N. Lewis. These visual representations were given the name Lewis electron dot diagrams.
Dot & Cross Diagrams. Dot and cross diagrams are diagrams that show the arrangement of the outer-shell electrons in an ionic or covalent compound or element. The electrons are shown as dots and crosses. In a dot and cross diagram: Only the outer electrons are shown. The charge of the ion is spread evenly which is shown by using .
The dots on the MgCl 2 Lewis diagram indicate it. The central magnesium atom in the MgCl 2 molecule is represented as follows: One core magnesium atom and two chlorine atoms make up the MgCl 2 molecule. As a result, in the MgCl2 Lewis structure (dot structure), the total number of outermost valence shell electrons accessible is 2 + 2 .
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”. Draw the dot and cross diagram with the outer shells overlapping. Then draw the shared electrons inside the overlapping section. Nitrogen gas occurs naturally as a diatomic molecule. The bond between the two nitrogen atoms is a triple bond. Again, draw the dot and cross diagram with the outer shells overlapping.
Magnesium Chloride. [H = 1, C = 6, M g = 12, C l = 17]. View Solution. Q2. Draw an electron dot diagram to show the formation of ammonium ion [Atomic number: N = 7 and H = 1]. . By drawing an electron dot diagram, show the lone pair effect leading to the formation of ammonium ion from ammonia gas and hydrogen ion.
magnesium chloride dot diagram High School Chemistry/Lewis Electron Dot Diagrams 5.3: Lewis Diagrams. Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. The kernel of the atom, i.e., the nucleus together with the inner electrons, is represented by the chemical symbol, and only the valence electrons are .Magnesium dichloride is a magnesium salt comprising of two chlorine atoms bound to a magnesium atom. It is a magnesium salt, an inorganic chloride, a magnesium halide and an inorganic magnesium salt. ChEBI. Magnesium chloride. An inorganic compound consisting of one magnesium and two chloride ions.
magnesium chloride dot diagram|High School Chemistry/Lewis Electron Dot Diagrams
PH0 · How to draw ionic bonding dot and cross diagrams
PH1 · How to draw dot and cross diagram of magnesium
PH2 · How to Draw the MgCl2 Lewis Dot Structure.
PH3 · How to Draw MgCl2 Dot & Cross Diagram Part 1 #science
PH4 · High School Chemistry/Lewis Electron Dot Diagrams
PH5 · Drawing Dot
PH6 · Dot Diagram Of Magnesium Chloride
PH7 · Bonding
PH8 · 9.2: Lewis Electron Dot Diagrams
PH9 · 1.4.5 Dot & Cross Diagrams